
Manasasri Muralidharan
The previous article briefed us on the bacterial origins of the CRISPR-Cas9 technology. It also gave us a glimpse into the Cas9 protein’s ability to edit DNA. In this article, we see how enterprising scientists created an astonishing gene-editing technology centred around Cas9.
Human beings transforming themselves by altering their DNA is a celebrated theme in science-fiction! Though humankind is yet to achieve on-the-go physical metamorphosis, real-life scientists have been changing DNA in the laboratory for decades. Unravelling the secrets hidden within our DNA has helped scientists unearth the causes of deadly diseases and find innovative cures for the same.
“Science is about solving puzzles,” states Dr Jennifer Doudna, a biochemist from the University of California (UC), Berkley. She is part of the two-woman team awarded the 2020 Nobel Prize in Chemistry to create an exact technology to hack DNA!
Unravelling the fabric of life
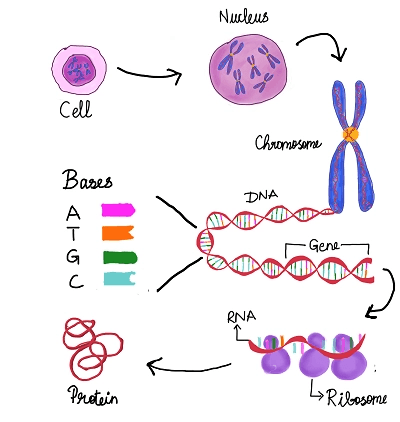
A peek into a cell’s supercomputer
Art by Manasa Muralidharan
The novel tech called CRISPR-Cas9 gene-editing was derived from the immune system of bacteria. It has the potential to rewrite and reprogram DNA. In a historically small time frame, CRISPR-Cas9 based editing or CRISPR editing has managed to mesmerise the scientific community. Since its conception in 2012, scientists have used this technology to correct parts of damaged DNA, attempt reversing the extinction of certain animals, and more.
To truly appreciate the genius of this technology, we need to look closer at the information stored in cells—the deoxyribonucleic acid or DNA functions as a cell’s supercomputer. Similar to computers using binary code (0 and 1) to write/store data, DNA uses unique letters to save and process information. Biological data is stored using four letters: A(Adenine), T(Thymine), G(Guanine), C(Cytosine). These letters or bases, when strung together in a particular combination, form a gene. Some genes transform into ribonucleic acid or RNA and code for proteins. Proteins monitor and facilitate numerous cellular tasks such as maintaining cell shape, repairing damaged DNA, transporting molecules, and more.
Cogs in the CRISPR machinery
The roles played by DNA, RNA, and proteins in the CRISPR immune system of bacteria were highlighted in this series’ first article. This immunity is mobilised against bacteriophages, which are viruses that attack and kill bacteria.
Due to the immense diversity in the components making up the CRISPR system, the defence response varies in different bacteria. Dr Emmanuelle Charpentier, a microbiologist, currently heading the Max Planck Institute for Infection Biology, unearthed a distinctive new player in the CRISPR system of Streptococcus pyogenes. They discovered that this flesh-eating bacteria spearheads its immune response using the trio of Cas9, a guide RNA (crisprRNA/crRNA), and a second unknown RNA. This discovery was astonishing because it proved for the first time that specific CRISPR systems used more than a single crRNA to recognise the attacking viral DNA.
Dynamic duo: Biological GPS and Genetic scissors
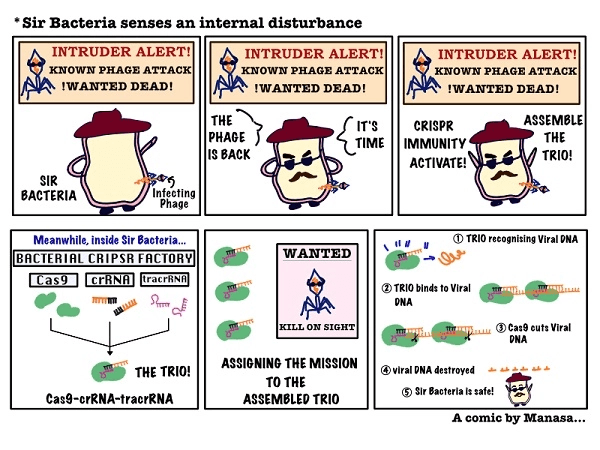
Streptococcal CRISPR Immunity in Action
A comic by Manasa Muralidharan
The new RNA was called tracrRNA (trans-acting RNA), and Dr Charpentier’s team showcased how it worked in tandem with crRNA. Both RNA strands acted as dual guides for Cas9, and together, the trio mounted their seek-and-destroy mission against viruses.
Soon after shining the spotlight on tracrRNAs, Dr Charpentier collaborated with Dr Jennifer Doudna to decipher how Cas9 functioned. Cas9 could pry open and make specific cuts on viral DNA when aided by the GPS-like actions of the crRNA-tracrRNA guide. This intrigued the researchers and they wondered if they could persuade Cas9 to cut other DNA by suitably altering the guiding GPS signal.
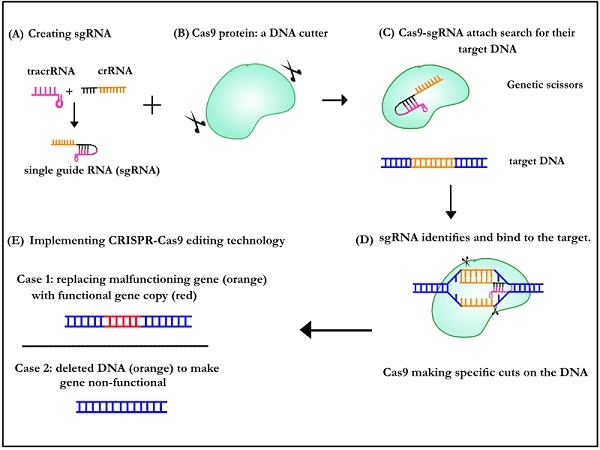
How do genetic scissors function?
Image made by Manasa Muralidharan
A new single-guide RNA (sgRNA) was created by melding together the crRNA-tracrRNA duo to study the above. The sgRNA was customisable and successfully allowed scientists to reprogram Cas9 to make cuts on the DNA of their choice! It paved the way for scientists to use this technology to redesign and overwrite any DNA.
Dr Charpentier and Dr Doudna’s ground-breaking research would eventually bag them the 2020 Nobel Prize in Chemistry. Upon the sturdy foundation of their innovation, new-age genome engineering innovations are being built. A few months after their work was published, Dr Feng Zhang, a biochemist at the Massachusetts Institute of Technology (MIT), applied their technology to edit human cells successfully. Envisioning the gold mine they were sitting atop, MIT applied for a fast-track patent granted to them in 2014. This began a dispute since the University of California’s application to patent the technology created by Dr Doudna and Dr Charpentier was still under review. The high stakes and the prospect of owning a biological cash cow led to a fierce patent war that is yet to be resolved.
Entering a better future one snip at a time?

Winners of the 2020 Nobel Prize in Chemistry: Dr Emmanuelle Charpentier (left) and Dr Jennifer Doudna (right) © Nobel Prize Outreach. Photo: Bernhard Ludewig
Ever since the new editing genie was let out of the bottle, researchers have furiously pitted the technology against multiple diseases caused due to DNA anomalies. The first series of clinical trials using CRISPR-based therapies to combat blood disorders such as sickle-cell anaemia and beta-thalassemia have shown immense promise.
Aiming to harness CRISPR editing’s spectacular potential, multiple companies and start-ups have mushroomed around the world. Though gene-editing technologies have pushed the boundaries of science, they have always been dogged by ethical and moral concerns. Let us address those concerns and celebrate the staggering biological innovations aided by CRISPR editing in this saga’s next and final story.
This article is a part of the series — The CRISPR Saga: How Bacteria equipped Humans to Transform the World





